Study Guide
Field 161: Chemistry
Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Sample Selected-Response Questions
The following reference materials will be available to you during the test:
Competency 0001
Structure of Matter
1. The electron geometry of nitrogen trifluoride, N F 3 , is best characterized as:
- bent linear.
- square planar.
- tetrahedral.
- trigonal pyramidal.
- Enter to expand or collapse answer. Answer expanded
- Correct Response: C. This item requires the candidate to demonstrate understanding of the concept of valence electron filling and orbital geometry. For a central atom with three bonded species, trigonal planar would be true for three bond pairs and no lone pairs. This does not, however, account for the unshared pair of electrons on nitrogen. This forces the bonds into a tetrahedral configuration.
Competency 0001
Structure of Matter
2. Use the video1 below to answer the question that follows. To play this video, click the Play icon ( ). You may also use the Pause (
). You may also use the Pause ( ), Stop (
), Stop ( ), and Replay (
), and Replay ( ) features. You may watch the video as many times as you wish.
) features. You may watch the video as many times as you wish.
The video begins with a glass petri dish resting on a support surface. A hand with tweezers holding a small piece of a solid material enters the frame. The piece of solid material is placed on the surface of the water and the hand quickly moves off the frame with the tweezers. In less than one second a bright flame is observed where the solid is touching the water. The material moves quickly across the surface of the water, emitting flame and smoke. It burns for several seconds, then extinguishes and a small blob of red glowing material is seen, which then breaks apart and appears to sink. Very little material is seen at the end sinking into the water.
The petri dish shown in the video contains water. The substance added to the water is most likely from which family in the periodic table?
- halogens
- alkali metals
- chalcogens
- alkaline earth metals
- Enter to expand or collapse answer. Answer expanded
- Correct Response: B. This item requires the candidate to analyze patterns in the physical and chemical properties of elements in terms of atomic structure. This video depicts the addition to water of a member of the alkali metal family of elements. Elements in this family are able to displace H two open parenthesis g close parenthesis from water, and the heat given off in this exothermic reaction ignites the H two open parenthesis g close parenthesis that is produced. The alkaline earth metals that do react with water will sink as they react but the alkali metals float.
Competency 0001
Structure of Matter
3. A chemistry teacher burns calcium in air, which produces calcium oxide and calcium nitride. Which Lewis electron-dot representation best depicts one of the two nitrogen ions in calcium nitride?
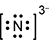 Response A is a diagram composed of a capital letter N with two dots above, two dots below, two dots to the left, and two dots to the right. All this has brackets around it and a small number three and negative sign beside it and slightly elevated.
Response A is a diagram composed of a capital letter N with two dots above, two dots below, two dots to the left, and two dots to the right. All this has brackets around it and a small number three and negative sign beside it and slightly elevated.
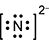 Response B is a diagram composed of a capital letter N with two dots above, two dots below, two dots to the left, and two dots to the right. All this has brackets around it and a small number two and a negative sign beside it and slightly elevated.
Response B is a diagram composed of a capital letter N with two dots above, two dots below, two dots to the left, and two dots to the right. All this has brackets around it and a small number two and a negative sign beside it and slightly elevated.
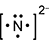 Response C is a diagram composed of a capital letter N with two dots above, one dot below, one dot to the left, and one dot to the right. All this has brackets around it and a small number two and a negative sign beside it and slightly elevated.
Response C is a diagram composed of a capital letter N with two dots above, one dot below, one dot to the left, and one dot to the right. All this has brackets around it and a small number two and a negative sign beside it and slightly elevated.
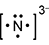 Response D is a diagram composed of a capital letter N with two dots above, one dot below, one dot to the left, and one dot to the right. All this has brackets around it and a small number three and a negative sign beside it and slightly elevated.
Response D is a diagram composed of a capital letter N with two dots above, one dot below, one dot to the left, and one dot to the right. All this has brackets around it and a small number three and a negative sign beside it and slightly elevated.
- Enter to expand or collapse answer. Answer expanded
- Correct Response: A. This item requires the candidate to demonstrate knowledge of the use of an electron-dot diagram to represent valence electron arrangement. Calcium nitride has the formula C a 3 N 2 . Nitrogen is in group 5A and has 5 valance electrons. In the case of a nitrogen ion in calcium nitride, an additional 3 electrons are gained from the calcium ions. This brings the total number of electrons to 8 for a given nitride ion in the electron-dot representation, in addition to the 3 minus charge, which is a result of the transfer of electrons.
Competency 0002
Properties of Matter
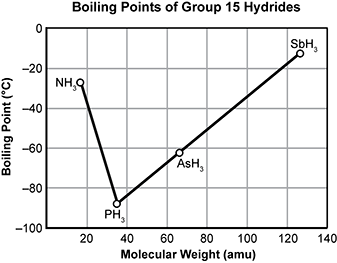
4. Use the graph below to answer the question that follows.
The graph is titled Boiling Points of Group fifteen Hydrides. The vertical axis of the graph is labeled Boiling Point in degrees Celsius. The scale is zero at the top down to negative one hundred at the bottom. The horizontal axis is labeled Molecular Weight in A M U and the scale goes from zero on the left to one hundred forty on the right. There are four points plotted on the graph and line segments connecting them. N H three, P H three, A S H three, and S B H three are all plotted on the graph. The three hydrides other than ammonia make a line segment from the region of high molecular weight and high boiling point to the region of low molecular weight and low boiling point. The graph then changes slope dramatically with ammonia having a much higher boiling point than the trend line would predict if it were continued.
The graph shows boiling points of hydrogen compounds of group 15 elements. Which statement best explains the deviation of ammonia from the trend of boiling points of the group's hydrides?
- The phosphine molecules have a much smaller radius than the ammonia molecules.
- The ammonia molecules are held together by hydrogen bonding, but molecules of the other hydrides are held together by weaker dipole-dipole interactions.
- The phosphine molecules have a much more ordered intermolecular structure than the ammonia molecules.
- The ammonia molecules are held together by stronger dipole-dipole forces, but weaker dipole-induced dipole forces hold molecules of the other hydrides together.
- Enter to expand or collapse answer. Answer expanded
- Correct Response: B. This item requires the candidate to demonstrate knowledge of the characteristics of different types of intermolecular forces present between given molecules. In the group 15 hydrides, the general trend in which a decrease in boiling point is associated with decreasing molecular weight is not observed for ammonia. This variation is the result of hydrogen bonding that takes place in N H 3 , because this type of intermolecular force requires a greater input of energy compared to dipole-dipole interactions in order to reach boiling point.
Competency 0002
Properties of Matter
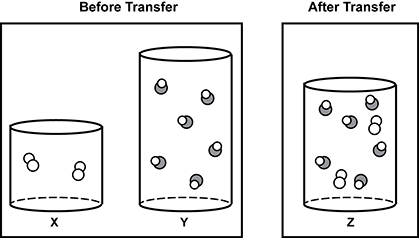
5. Use the diagram below to answer the question that follows.
The diagram shows two conditions. On the left is two cylinders shown in cutaway drawings to be able to see the contents of the cylinders. One cylinder is labeled X and contains what appears to be two molecules of a gas. The diagram of the molecules is made of two identical circles joined. On the right is a taller cylinder with same diameter containing what appears to be six molecules of a different gas, which is drawn as a set of two joined circles, one larger and shaded and one white. These two cylinders are in a group labeled before transfer. There is another group labeled after transfer. This is a single cylinder whose height is somewhere between the other two cylinders. It is labeled Z and contains all of the drawings from the first two cylinders, for a total of eight molecule drawings in the cylinder.
The diagram shows three cylinders of gases. Cylinder X has a volume of 1.50 L and contains a gas at a pressure of 0.860 a t m . Cylinder Y has a volume of 3.10 L and contains a gas at a pressure of 1.28 a t m . The gases inside cylinders X and Y are transferred into cylinder Z, which has a volume of 2.20 L. All cylinders are held at a constant temperature. Which value represents the approximate pressure of the combined gases in cylinder Z?
- 1.07 a t m
- 2.14 a t m
- 2.39 a t m
- 4.60 a t m
- Enter to expand or collapse answer. Answer expanded
- Correct Response: C. This item requires the candidate to apply gas laws and mathematical thinking to analyze data pertaining to the relationship between volume and pressure. In this problem, Boyles Law (P1V1 = P2V2) can be used to find the partial pressure of each gas, and then Daltonís Law of Partial Pressures can be used to find the combined pressures of the gases in cylinder Z. For cylinder X, 0.860 a t m times 1.50 L = P2 times 2.20 L results in a partial pressure of 0.59 a t m , while for cylinder Y, 1.28 a t m times 3.10 L = P2 times 2.20 L results in a partial pressure of 1.80 a t m . The final pressure in cylinder Z is thus 0.59 a t m + 1.80 a t m = 2.39 a t m .
Competency 0003
Chemical Reactions
6. Use the chemical equation below to answer the question that follows.
3 H 2 S open parenthesis A Q close parenthesis plus K 2 C R 2 O 7 open parenthesis A Q close parenthesis plus 4 H 2 S O 4 open parenthesis A Q close parenthesis reaction arrow 3 S open parenthesis S close parenthesis plus C R 2 open parenthesis S O 4 close parenthesis subscript 3 open parenthesis A Q close parenthesis plus K 2 S O 4 open parenthesis A Q close parenthesis plus seven H 2 O open parenthesis L close parenthesis
In the equation shown, which set of values best describes the change in the oxidation number of sulfur from reactants to products, respectively?
- minus 12 to minus 2
- minus 6 to 3
- minus 4 to minus 4
- minus 2 to 0
- Enter to expand or collapse answer. Answer expanded
- Correct Response: D. This item requires the candidate to demonstrate understanding of oxidation-reduction reactions. In this equation, sulfur in the form of the sulfate ion S O four two minus acts as a spectator ion and does not take part in the reaction. Sulfur on the reactant side of the equation, in the form of H 2 S , has an oxidation number of minus 2 (hydrogen is +1 and two hydrogen atoms are present). On the product side of the reaction, sulfur is present as a free element with the corresponding oxidation number of 0.
Competency 0003
Chemical Reactions
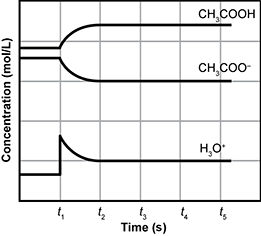
7. Use the chemical equation and graph below to answer the question that follows.
C H 3 C O O H open parenthesis A Q close parenthesis plus H two O open parenthesis L close parenthesis two way reaction arrow H 3 O one plus open parenthesis A Q close parenthesis plus C H 3 C O O one minus open parenthesis A Q close parenthesis
The graph presented has a vertical axis labeled as concentration in moles per liter. The horizontal axis is labeled time in seconds. There is no scale on the vertical axis. The horizontal axis is marked in 5 apparently equal increments marked with the letter T and a subscript number. They are T one, T 2, T 3, T 4, and T 5.
The graph has 3 plots connected with a continuous curve. 2 are close together and near the top, the last stands alone near the bottom.
The first, labeled C H 3 C O O H, begins with a straight horizontal segment until T one, then it curves upward until T 2 where it becomes horizontal again. It remains horizontal. The second, labeled C H 3 C O O one minus, is similar to the first, but inverted. It begins below the first with a straight horizontal segment until T one, then it curves downward until T 2 where it becomes horizontal again. It remains horizontal. The final, labeled H 3 O one plus, begins with a straight horizontal segment. At T one there is a spike, with a short vertical segment, then the plot curves downward and becomes horizontal like plot 2.
Which statement explains the change in the concentrations of products and reactants between t1 and t3?
- The quantities of acetic acid and acetate change until they reestablish an equimolar balance.
- The buffering capacity of acetic acid increases before the system stabilizes.
- The concentration of acetate is reduced to the point that its ability to react with acids is severely limited.
- The increase in hydronium ion concentration shifts the equilibrium toward the production of acetic acid.
- Enter to expand or collapse answer. Answer expanded
- Correct Response: D. This item requires the candidate to demonstrate knowledge of the factors that affect chemical equilibrium. The addition of hydronium ions ( H 3 O + ) at t1 causes the equilibrium position of the reaction to shift to the left. Specifically, an increase in the amount of acetic acid ( C H 3 C O O H ) occurs because the additional hydronium ions combine with the acetate ions ( C H 3 C O O H minus) to form additional molecules of acetic acid ( C H 3 C O O H ). While the concentration of acetate ion is reduced, it is not to the point where its ability to react with acids would be severely limited.
Competency 0004
Energy
8. Use the passage below to answer the question that follows.
Students are asked to explore the thermal properties of different materials. They examine a plastic cup, a paper cup, and an aluminum cup. Each cup has the same mass and, throughout the investigation, the external conditions remain the same. The students determine that the aluminum cup feels like the coldest of the three materials. They measure the three cups with a temperature probe and find that the cups are the same temperature. The teacher then asks the students to place an ice cube into each cup in order to investigate which cup's material will melt the ice the fastest.
Which statement most accurately identifies the expected result of the investigation and the explanation for that result?
- The ice cubes in all the cups will melt at the same rate because all of the cups' materials contain an equivalent amount of heat.
- The paper cup's ice cube will melt the fastest because the paper's texture allows more contact with the ice cube's surface area than the other materials.
- The aluminum cup's ice cube will melt the fastest because aluminum conducts energy more efficiently than the other materials.
- The cup with the fastest melting ice cube depends upon how the cups are heated externally because some materials heat more rapidly than other materials.
- Enter to expand or collapse answer. Answer expanded
- Correct Response: C. This item requires the candidate to apply scientific principles related to energy to evaluate energy transfer. Aluminum is a better thermal conductor than either paper or plastic. Consequently, ice melts faster in the aluminum cup because heat from the surrounding environment is able to flow more readily into the aluminum cup and to the ice cube, causing it to melt.
Competency 0004
Energy
9. Use the table below to answer the question that follows.
Row Delta G Delta H Delta S 1 minus + + 2 minus minus + 3 + minus minus 4 + + minus
When pellets of sodium hydroxide are added to water, the pellets dissolve and the temperature of the resulting mixture rises. Which row in the table best represents Delta G, Delta H, and Delta S for the process?
- Row 1
- Row 2
- Row 3
- Row 4
- Enter to expand or collapse answer. Answer expanded
- Correct Response: B. This item requires the candidate to demonstrate understanding of the concepts of entropy and free energy and predict the spontaneity of given reactions. This reaction is an exothermic reaction because heat is released as the sodium hydroxide dissolves in water. In exothermic reactions, the enthalpy of the system is lowered so Delta H is less than 0. In addition, as the sodium hydroxide dissolves, the amount of disorder of the system is increased so Delta S is greater than 0 when sodium hydroxide dissolves in water.
Acknowledgments
1Ukrainian Media Archive. Sodium metal reacting with liquid. Credit Line: Ukrainian Media Archive. Shutterstock Ukrainian Media Archive.