Study Guide
Field 161: Chemistry
Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Review the special character palette available for science CSTs:
Sample Constructed-Response Item 1
Competency 0005
Pedagogical Content Knowledge
Use the following New York State P to 12 Science Learning Standard to complete the assignment below.
Using your pedagogical and content knowledge of chemistry, write a response of approximately 400 to 600 words in which you:
- identify a specific learning goal for the lesson;
- describe how you will assess student readiness for the lesson's learning goal;
- describe an appropriate and effective three-dimensional (i.e., disciplinary core idea, crosscutting concept, science or engineering practice) instructional strategy that you would use to help students meet the learning goal;
- explain how the instructional strategy you described will connect students' prior understanding (e.g., cultural relevancy, real-world experience) to the new knowledge related to the learning goal;
- describe a modification to the instruction that you would use and explain how it would address the strengths and/or needs of all students; and
- describe an appropriate and effective assessment related to the learning goal, in order to evaluate and promote learning and growth for all students.
Learning Standard
You are planning instruction for a middle school chemistry class. The lesson that you are preparing to teach will address the following standard from the New York State P to 12 Science Learning Standards.1
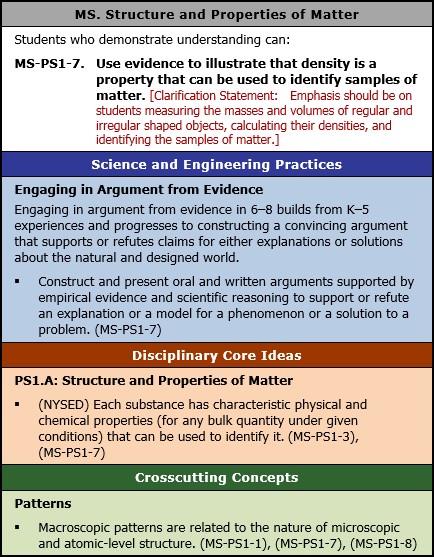
MS. Structure and Properties of Matter
Students who demonstrate understanding can:
MS-PS1-7. Use evidence to illustrate that density is a property that can be used to identify samples of matter. [Clarification Statement: Emphasis should be on students measuring the masses and volumes of regular and irregular shaped objects, calculating their densities, and identifying the samples of matter.]
Science and Engineering Practices
Engaging in Argument from Evidence
Engaging in argument from evidence in 6 to 8 builds from K to 5 experiences and progresses to constructing a convincing argument that supports or refutes claims for either explanations or solutions about the natural and designed world.
- Construct and present oral and written arguments supported by empirical evidence and scientific reasoning to support or refute an explanation or a model for a phenomenon or a solution to a problem. (MS-PS1-7)
Disciplinary Core Ideas
PS1.A: Structure and Properties of Matter
- (NYSED) Each substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. (MS-PS1-3), (MS-PS1-7)
Crosscutting Concepts
Patterns
- Macroscopic patterns are related to the nature of microscopic and atomic-level structure. (MS-PS1-1), (MS-PS1-7), (MS-PS1-8)
Sample Strong Response to Constructed-Response Item 1
The goal of this lesson is to have students calculate the density of a variety of objects and use the calculated density values as evidence to identify the substances. Students will then sort a group of objects according to composition.
After the initial demonstration that will engage student interest, the teacher will assess readiness. The teacher will ask for some predictions about whether common objects will sink or float in water. During this questioning the teacher will gather an informal understanding of the student's readiness. Students will also have to perform basic density calculations. For this activity regular geometric shapes are used.
To reach the learning goal this lesson will start with a demonstration of objects with variations in density. A large tank filled with water will be uncovered, arranged so students can gather around. A few questions about things that sink or float will be used to introduce the topic. The teacher will then reveal a watermelon and an ear of corn. Students will have a moment to hold the items, comparing their mass. The teacher will then have a student drop the corn into the tank as the teacher drops the watermelon in. Students will see the watermelon floating and the corn on the bottom of the tank. A brief discussion will follow about why one floats. Next, pairs of two-inch cubes of wood, one pine and one ebony, are distributed. Students will be instructed to qualitatively compare the mass of each block by switching them back and forth in their hands. If a student is unsure they can use a balance to get an actual mass. Students will then test the blocks in the tank and see that one sinks and one floats. A brief question period follows, where students will respond about objects of the same size but different mass and therefore different density. Next, students will be given a set of three spheres of different materials, all painted white to obscure their composition. Students will calculate density of the objects by finding volume and mass. A table will be provided with density values for materials that includes the wood, plastic and metal the spheres are made of. Students will have to identify the composition of each of the spheres. A brief discussion will follow to check student's understanding.
Student's prior knowledge will be connected through question and answer and discussion throughout the initial demo and following the main activity. After the density determination activity students will break into small groups to discuss the results. A few cards with conversation topics will be distributed to aid the discussion. The teacher will do an additional demonstration showing one bar of soap that floats and one that sinks. Students will be asked to describe both surprising and predictable examples in their lives of density and flotation.
To modify the instruction additional examples can be demonstrated. A bowling ball floating can be demonstrated, which is a surprising outcome. Various bars of soap can be placed in the tank, which will tie in the earlier discussion topic.
For assessment, students will be asked to be detectives in identifying a substance. The story of Archimedes determining the composition of a gold crown by displacement will be shared, then students will have to determine the density of a piece of iron pyrite to determine if it is real gold. The density will be determined and compared to known density values. A determination of the identity of this substance based on the evidence collected will be made. The written output of this step will be peer reviewed and revised in a subsequent class period prior to completion.
Sample Constructed-Response Item 2
Competency 0005
Pedagogical Content Knowledge
Use the following New York State P to 12 Science Learning Standard to complete the assignment below.
Using your pedagogical and content knowledge of chemistry, write a response of approximately 400 to 600 words in which you:
- identify a specific learning goal for the lesson;
- describe how you will assess student readiness for the lesson's learning goal;
- describe an appropriate and effective three-dimensional (i.e., disciplinary core idea, crosscutting concept, science or engineering practice) instructional strategy that you would use to help students meet the learning goal;
- explain how the instructional strategy you described will connect students' prior understanding (e.g., cultural relevancy, real-world experience) to the new knowledge related to the learning goal;
- describe a modification to the instruction that you would use and explain how it would address the strengths and/or needs of all students; and
- describe an appropriate and effective assessment related to the learning goal, in order to evaluate and promote learning and growth for all students.
Learning Standard
You are planning instruction for a high school chemistry class. The lesson that you are preparing to teach will address the following standard from the New York State P to 12 Science Learning Standards.2
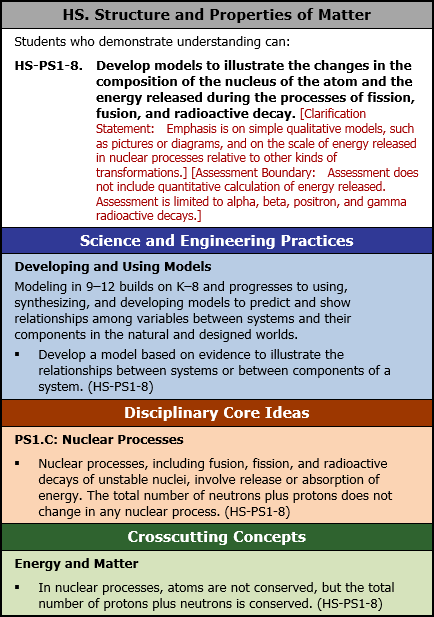
HS. Structure and Properties of Matter
Students who demonstrate understanding can:
HS-PS1-8. Develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the processes of fission, fusion, and radioactive decay. [Clarification Statement: Emphasis is on simple qualitative models, such as pictures or diagrams, and on the scale of energy released in nuclear processes relative to other kinds of transformations.] [Assessment Boundary: Assessment does not include quantitative calculation of energy released. Assessment is limited to alpha, beta, positron, and gamma radioactive decays.]
Science and Engineering Practices
Developing and Using Models
Modeling in 9 to 12 builds on K to 8 and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds.
- Develop a model based on evidence to illustrate the relationships between systems or between components of a system. (HS-PS1-8)
Disciplinary Core Ideas
PS1.C: Nuclear Processes
- Nuclear processes, including fusion, fission, and radioactive decays of unstable nuclei, involve release or absorption of energy. The total number of neutrons plus protons does not change in any nuclear process. (HS-PS1-8)
Crosscutting Concepts
Energy and Matter
- In nuclear processes, atoms are not conserved, but the total number of protons plus neutrons is conserved. (HS-PS1-8)
Sample Strong Response to Constructed-Response Item 2
Students will understand the process of nuclear decay, identify and label the products of nuclear decay, and draw a model of common nuclear decay processes.
Assess Prior Knowledge
1. Question and answer session
2. Common types of radiation
a. Particle emissions that change nucleus
- Alpha
- Beta
- Positron
b. Energy: does not change nucleus
- Gamma
Video on Henri Becquerel's Discovery of Radioactivity to Engage Students
1. Mysterious exposure of photographic plates
a. Sealed envelope
b. No source of light
2. Working with uranium salts
a. X-rays recently discovered
b. Uranium shown to emit invisible rays
- Initially thought to be a result of absorbing sunlight
- Shown to emit radiation without sunlight exposure
3. Went on to work with other types of radiation
Student Discussion and Extension
1. What was surprising about this discovery?
2. What steps would you take to further explore this?
a. What can radiation penetrate?
b. Do magnetic and electric fields affect radiation?
c. What could this penetrating radiation be useful for?
d. What technologies use this in the modern day?
Connect with Prior Learning
1. Discussion on radiation and radioactive material they have heard of
a. Medical use
- Imaging
- Safety requirements
b. Radioactive waste
- Long life
- Challenges of disposal
c. Military use
d. Radon in homes
3-D Lesson
1. Identify Radiation Types
a. Show example of notation for nuclei
- Total number of nucleons
- Number of protons
- Find neutrons from the difference
b. Give example of alpha and beta decays
- Show change in nuclides and protons for alpha
- Change of a neutron to a proton for beta
2. Model Types of Radioactive Decay and Products
a. Have students determine type of decay
- Give before and after nuclei
- Determine what changed
- Determine what kind of radiation emitted
b. Have students determine decay product
- Give a starting nucleus and type of decay
- Students determine number of protons and neutrons
- Determine identity of product
3. Model a Decay Series
a. Pairs of students get a card with a step in decay series.
- Card has original nucleus and type of decay.
- Product not listed
- Some duplication is possible depending on size of group.
b. Students will determine decay product for their card.
- Reference chart available for types of radiation.
- Uranium decay series has many steps.
c. Students will then work with other groups and match product with starting material.
- Each step will have unique starting nucleus and product.
- If a match is not found the teacher will be consulted.
- Students can line up or post on board with magnets.
- Entire decay series will be modeled.
d. Students will share their step with the class.
- State starting material and product.
- State type of emission.
- State what part of nuclear identity changed.
- State how proton to neutron ratio changed.
e. Students will be asked to identify a pattern.
- How does ratio change?
- What is difference between starting material and stable product?
Modifying Instruction
1. Additional examples of decay series
a. Actinium and thorium can be used and modeled.
- Compare and contrast steps to uranium series.
- What are ratios for starting materials?
- What are ratios for stable products?
2. Discussion on how materials are used in medical imaging
a. Ingest the isotope
b. Have scan performed
c. Dosage
d. Short half-life vs. long life
Assessing Student's Understanding
1. Report on results of activity
2. Describe pattern in nuclide ratio
3. Peer review and revise
Performance Characteristics for a Constructed-Response Item
The following characteristics guide the scoring of the response to a constructed-response item.
| Completeness | The degree to which the response addresses all parts of the assignment |
|---|---|
| Accuracy | The degree to which the response demonstrates the relevant knowledge and skills accurately and effectively |
| Depth of Support | The degree to which the response provides appropriate examples and details that demonstrate sound reasoning |
Score Scale for a Constructed-Response Item
A score will be assigned to the response to a constructed-response item according to the following score scale.
| Score Point | Score Point Description |
|---|---|
| 4 |
The "4" response reflects a thorough command of the relevant knowledge and skills:
|
| 3 |
The "3" response reflects a general command of the relevant knowledge and skills:
|
| 2 |
The "2" response reflects a partial command of the relevant knowledge and skills:
|
| 1 |
The "1" response reflects little or no command of the relevant knowledge and skills:
|
| U | The response is unscorable because it is unrelated to the assigned topic or off task, unreadable, written in a language other than English or contains an insufficient amount of original work to score. |
| B | No response. |
Acknowledgments
1From the New York State Education Department. New York State P–12 Science Learning Standards (2018). Internet. Available from http://www.p12.nysed.gov/ciai/mst/sci/documents/p-12-science-learning-standards.pdf; accessed 23 June 2018.